Vinegar battery science project
Vinegar Battery Science Project. Whenever two different metallic substances like Zinc and copper are dipped in an acidic medium electrolyte then a chemical reaction happens between the metals and the electrolyte which further generates a charge. 5 pieces of copper wire. Your science loving kiddos from Kindergartners Grade 1 grade 2 grade 3 grade 4 grade 5 and grade 6 will love these battery experiments. Theyre the sites in a battery where moving electrons the current enter and then leave the battery.
 How To Make Ice Cube Tray Vinegar Battery Hypothesis And Explanation Of Experiment Step By Step From electricalfundablog.com
How To Make Ice Cube Tray Vinegar Battery Hypothesis And Explanation Of Experiment Step By Step From electricalfundablog.com
The working principle of our Ice Cube Tray Vinegar Battery is very simple. This how to make a battery science project provides kids with a simple inexpensive way to create their own homemade battery experiment using materials that are likely already in their home pennies aluminum foil paper towels vinegar and duct tape. Whenever two different metallic substances like Zinc and copper are dipped in an acidic medium electrolyte then a chemical reaction happens between the metals and the electrolyte which further generates a charge. If you want to generate a larger charge then connect few similar cells together in series. By stacking additional matboards and sanded pennies youve created a battery which is a series of electrochemical cells. So when we add the colored vinegar to the colored chalk the mixture makes that cool fizzing reaction.
If you want to generate a larger charge then connect few similar cells together in series.
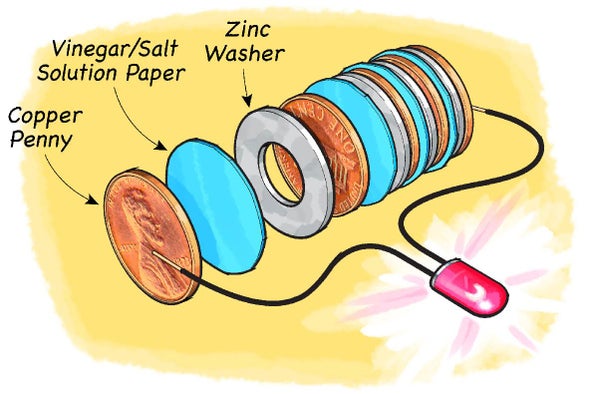
Explore the world of chemistry with these fun battery experiments for kids. Take two glasses and fill them with vinegar. Baking soda and vinegar react with each other because of an acid-base reaction. In this activity you will create a basic homemade battery with just construction paper vinegar salt and a handful of pennies and washersand prove it works by lighting an LED. Expel the negative link from the battery and afterward evacuate the positive link. Each zinc-matboard-copper stack represents one individual cell.
 Source: youtube.com
Source: youtube.com
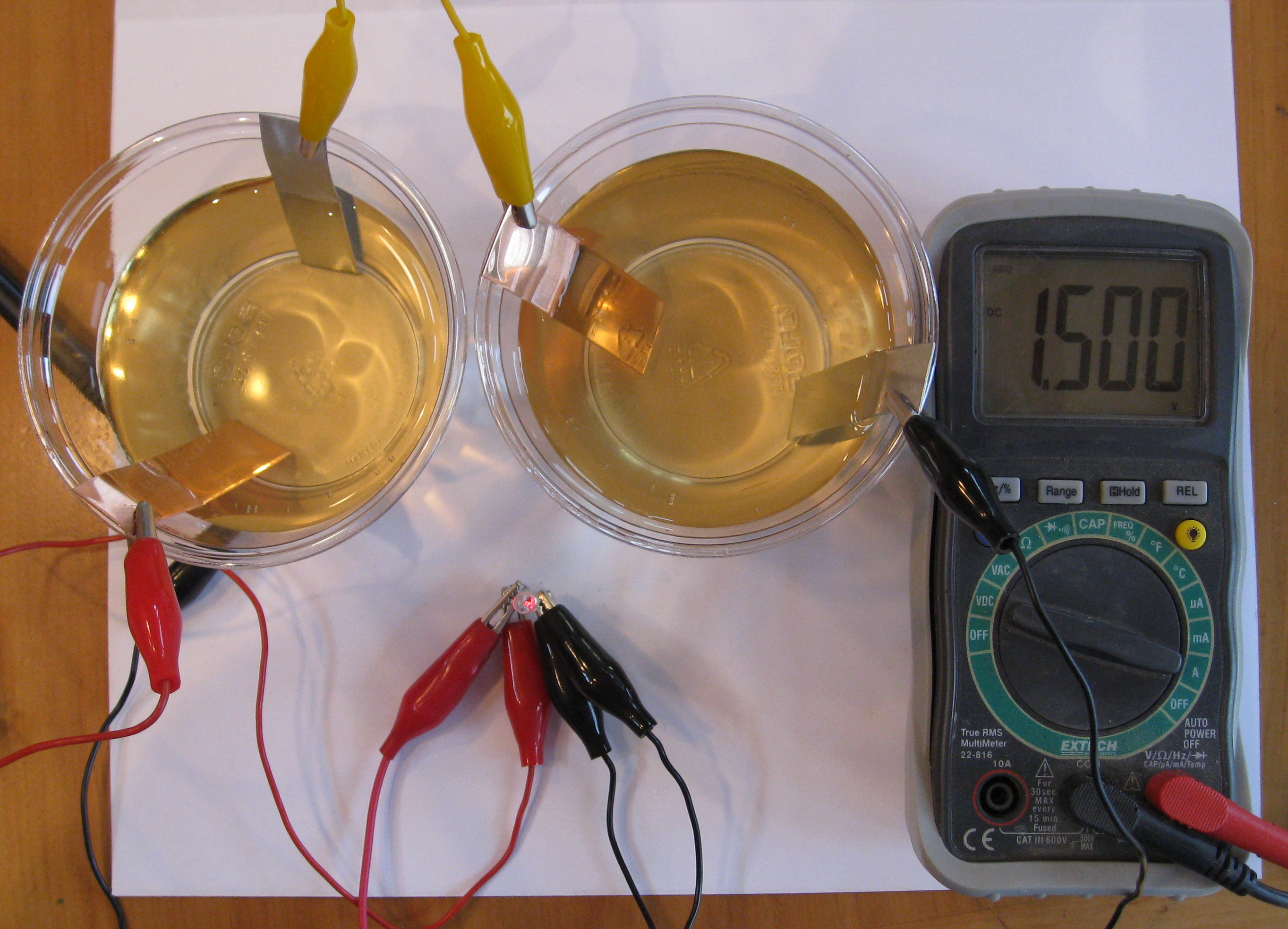
Each zinc-matboard-copper stack represents one individual cell. By stacking additional matboards and sanded pennies youve created a battery which is a series of electrochemical cells. This strip will make it easier to connect the multimeter later. Place the aluminum strip in the middle of your plate. The matboard soaked in salty vinegar water serves as the electrolyte between the two terminals.
 Source: youtube.com
Source: youtube.com
Vinegar Two glasses Two zinc strips Two copper strips Connecting wires LED Bulb Procedure. By stacking additional matboards and sanded pennies youve created a battery which is a series of electrochemical cells. The Ice Tray Battery is the ultimate when it comes to kids science activities using vinegar. Baking soda is a bicarbonate and vinegar is an acetic acid. Simple household items throw their current-conducting properties into the pot to create a basic version of the batteries you buy at the store.
 Source: sci-toys.com
Source: sci-toys.com
Spot a blend of preparing pop and white vinegar around the terminal post and let it misfire up. Whenever two different metallic substances like Zinc and copper are dipped in an acidic medium electrolyte then a chemical reaction happens between the metals and the electrolyte which further generates a charge. The matboard soaked in salty vinegar water serves as the electrolyte between the two terminals. The working principle of our Ice Cube Tray Vinegar Battery is very simple. Wipe any thick oil off with a perfect cloth.
 Source: pinterest.com
Source: pinterest.com
Heating pop and vinegar are magnificent for cleaning battery terminals. By stacking additional matboards and sanded pennies youve created a battery which is a series of electrochemical cells. Create simple circuits a simple powered motor and a robot from one of sciences greatest inventions. This strip will make it easier to connect the multimeter later. Baking soda and vinegar react with each other because of an acid-base reaction.
 Source: cool-solar-stuff.com
Source: cool-solar-stuff.com
Expel the negative link from the battery and afterward evacuate the positive link. The two metal components are electrodes. The Ice Tray Battery is the ultimate when it comes to kids science activities using vinegar. Heating pop and vinegar are magnificent for cleaning battery terminals. This well-known activity demonstrates the reactions between acids and bases.
 Source: hilaroad.com
Source: hilaroad.com
The working principle of our Ice Cube Tray Vinegar Battery is very simple. The two metal components are electrodes. Simple household items throw their current-conducting properties into the pot to create a basic version of the batteries you buy at the store. Voltaic batteries turn chemical energy into the electrical energy we use to power electronics. Fit the balloon over the top shake the baking soda down into the vinegar and watch the balloon inflate.
 Source: electricalfundablog.com
Source: electricalfundablog.com
Your science loving kiddos from Kindergartners Grade 1 grade 2 grade 3 grade 4 grade 5 and grade 6 will love these battery experiments. By stacking additional matboards and sanded pennies youve created a battery which is a series of electrochemical cells. The zinc is in the galvanization on the nail galvanization prevents rust and in the copper of the wire. 5 pieces of copper wire. This well-known activity demonstrates the reactions between acids and bases.
 Source: youtube.com
Source: youtube.com
Place the aluminum strip in the middle of your plate. Whenever two different metallic substances like Zinc and copper are dipped in an acidic medium electrolyte then a chemical reaction happens between the metals and the electrolyte which further generates a charge. Theyre the sites in a battery where moving electrons the current enter and then leave the battery. Baking soda and vinegar react with each other because of an acid-base reaction. Simple household items throw their current-conducting properties into the pot to create a basic version of the batteries you buy at the store.
 Source: electricalfundablog.com
Source: electricalfundablog.com
Spot a blend of preparing pop and white vinegar around the terminal post and let it misfire up. The science behind it. The acid comes from the vinegar you poured into the ice tray. With this science experiment student can create their own voltaic batteries using ordinary items. Create simple circuits a simple powered motor and a robot from one of sciences greatest inventions.
 Source: electricalfundablog.com
Source: electricalfundablog.com
This strip will make it easier to connect the multimeter later. So when we add the colored vinegar to the colored chalk the mixture makes that cool fizzing reaction. The colors change due to adding two colors together to make another color. Therefore the reaction this creates is carbon-dioxide. A fun and great project to try.
 Source: scientificamerican.com
Source: scientificamerican.com
Voltaic batteries turn chemical energy into the electrical energy we use to power electronics. Spot a blend of preparing pop and white vinegar around the terminal post and let it misfire up. Wipe any thick oil off with a perfect cloth. Create simple circuits a simple powered motor and a robot from one of sciences greatest inventions. The acid comes from the vinegar you poured into the ice tray.
 Source: exploratorium.edu
Source: exploratorium.edu
This well-known activity demonstrates the reactions between acids and bases. The working principle of our Ice Cube Tray Vinegar Battery is very simple. Baking soda and vinegar react with each other because of an acid-base reaction. The colors change due to adding two colors together to make another color. Spot a blend of preparing pop and white vinegar around the terminal post and let it misfire up.
 Source: youtube.com
Source: youtube.com
This well-known activity demonstrates the reactions between acids and bases. Baking soda and vinegar react with each other because of an acid-base reaction. This strip will make it easier to connect the multimeter later. You will build your battery on top. More specifically since vinegar is almost all water the acid comes from the 4-8 of acetic acid dissolved in it.
 Source: pinterest.com
Source: pinterest.com
A few pennies and nickels small paper-towel squares a vinegar-salt solution and an aluminum strip is all you need to create a coin battery. Chances are good you probably did easy science experiments like this when you were in school yourself. Baking soda and vinegar react with each other because of an acid-base reaction. Baking soda is a bicarbonate and vinegar is an acetic acid. Each zinc-matboard-copper stack represents one individual cell.
 Source: hilaroad.com
Source: hilaroad.com
Explore the world of chemistry with these fun battery experiments for kids. This well-known activity demonstrates the reactions between acids and bases. This how to make a battery science project provides kids with a simple inexpensive way to create their own homemade battery experiment using materials that are likely already in their home pennies aluminum foil paper towels vinegar and duct tape. Whenever two different metallic substances like Zinc and copper are dipped in an acidic medium electrolyte then a chemical reaction happens between the metals and the electrolyte which further generates a charge. Wipe any thick oil off with a perfect cloth.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title vinegar battery science project by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





